Temperature And Ice
Kevin Rose | Miami University (temperature and ice)
The Formation of Lake Ice
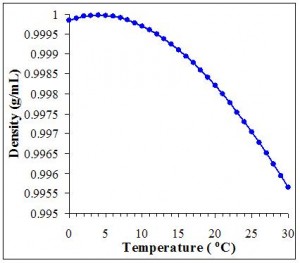
The density of water is greatest at about 4° C and decreases at temperatures both above and below this point. (Modified from 4).
Water has some unique thermal properties. For most substances, density increases as temperature decreases. However, peak density of water occurs in water at around 4oC and decreases at temperatures both above and below this (see Figure 1). Because ice is less dense than the underlying water, it floats. Without this unique property, life on Earth would probably not exist. Since ice floats, it forms a barrier and insulating layer between the warmer underlying water and the colder air above a lake. If ice were denser than water in the way that the solids of most materials are denser than their liquid states, ice would sink. Sinking ice would expose the underlying water to cold air temperatures which would again freeze the water and sink. Eventually this process would continue until a lake was completely frozen solid. Since most organisms cannot survive being frozen, this would create big problems for most aquatic species! Only in rare cases do lakes actually freeze solid – generally this only happens where lakes are very shallow and the climate gets extremely cold. Because the maximum density of water occurs at 4oC, the hypolimnion – the dense, bottom water layer of lakes – usually stays around 4oC and doesn’t change much throughout the year.
What happens under the ice of lakes? Water under the ice typically stays very cold, but above freezing. Most lakes that form ice at the surface stay at about 4oC most of the winter months. Most lake organisms (e.g., phytoplankton/algae, zooplankton, and most fish) are cold-blooded, meaning their body temperature varies with the temperature of the water. Therefore, the metabolism of organisms living under the ice slows way down compared to warmer summer months. Ice forms a barrier to the flux of gasses and energy flowing into and out from lakes. In some cases, oxygen levels can fall under the ice which can harm the organisms that live there. However, algae continue to photosynthesize and produce oxygen as long as there is enough light, so oxygen is being created as it is being consumed.
Ice as a Climate Indicator
Lake temperatures and ice formation on lakes have been recorded throughout history. Calendar dates of freezing and thawing of lakes has been recording in writings for hundreds of years. For example, Lake Suwa in Japan has an ice record dating back more than 550 years1. Often, these early measurements were made for religious, cultural, or practical reasons concerning transportation over ice vs. open water. In many cases, these records exist before modern thermometers. Scientists can use these records to estimate past climate and weather conditions, using lakes as sentinels of broader environmental and climate change. Year to year variability can be high due to local weather conditions, but long term records yield insightful data. Data show that ice is forming on lakes later and breaking up earlier than it did decades and centuries ago1. These simple records of ice provide a natural indicator of climate change that can be widely observed.
Does Ice Affect Climate Warming?

The Baikal Seal is the only freshwater species of seal on the planet. It depends on ice to build shelters on Lake Baikal, but climate change is rapidly reducing the duration of ice cover on the lake. (Image from 5).
Water has a high specific heat capacity, about 3,000 times greater than air2. Thus, it takes much more heating or cooling to alter the temperature of a lake compared with the air. While changes in climate and weather may alter the air temperature rapidly, changes in water temperature usually occur slower. Slow changes in water temperature are especially common in larger lakes due to their high thermal mass. However, records show that in some lakes water temperature is changing faster than air temperatures – and ice may be the reason.
While water generally warms and cools slower than the atmosphere, research on lakes, temperature, and global climate change show this is not always true. A long-term data set collected from 1945 to present day on Lake Baikal in Russia shows that the water temperature is actually increasing about twice as fast as the air temperature3! Lake Baikal is the deepest, largest (by volume), and oldest lake in the world; because of its huge size, it has a large thermal mass. Additionally, Lake Baikal is home to some unique animals, including the only species of freshwater seal in the world. This seal depends on the lake’s water temperature and ice conditions to survive. It’s been hypothesized that the increase in temperature is due to changes in ice on the lake. Affected by global climate change, ice now forms on the lake later in the year and disappears earlier in the year than it used to. The change in ice alters the color of the lake surface — ice and snow cover are generally white, while the lake water itself is a deep blue. The change in color along with changes in ice alters the heat absorbed from solar radiation, thereby increasing the rate of long-term warming of the world’s largest lake.
Sources:
- Magnuson, J.J. et al., 2000, Historical Trends in Lake and River Ice Cover in the Northern Hemisphere, Science
- Molles, M.C., 2002, Ecology Concepts and Applications, New York
- Hampton, S.E. et al., 2008, Sixty years of environmental change in the world’s largest freshwater lake – Lake Baikal, Siberia, Global Change Biology
- Vallentyne J.R., 1957, Principles of Modern Limnology, American Scientist
- Lake Baikal: The Pearl of Russia Image Gallery. Pacific Environment. Retrieved online at: https://www.flickr.com/photos/pacific_environment/847100409/